全部商品 / LEICA 顯微影像系統與光學組件 / LEICA THUNDER 顯微影像系統
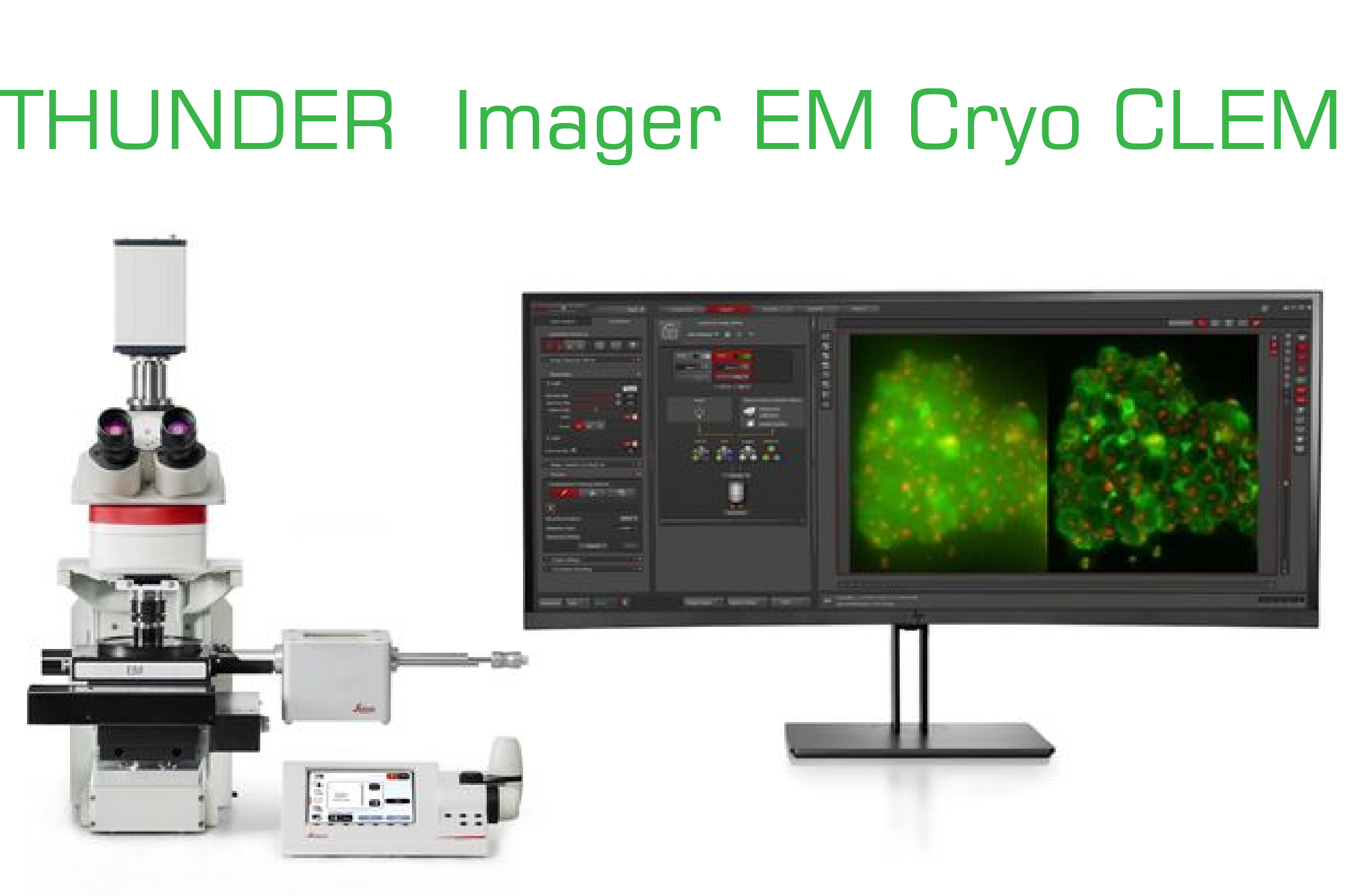
冷凍電顯與光學相干(EM Cryo CLEM) 之顯微影像處理系統
最近利用諾貝爾得獎技術的低溫電子顯微鏡的分子成像技術 (cryo EM), 倍受重視, 此技術可以動生物樣本的結構觀察, 實現到原子尺度的解析度,前所未有地識別出了蛋白質中的單個原子, 了解蛋白質的工作原理,這是過去其他成像技術(如 X-射線晶體學)無法檢測到的. 結合光學顯微鏡技術與低溫電顯的聯合影像技術 (CLEM), 將會使原子解析等級的觀察技術更上一層樓 !
THUNDER Imager EM Cryo CLEM 是新世代的低溫光學顯微鏡結合數位光電澄清影像與提升解析的解決方案, 利用 THUNDER, 可以把受測樣本區通過高解析度的影像澄清處理、即時去除焦平面外的模糊信號,從而對樣本細胞結構的特定選擇區域, 作更精確的確認須受低溫電顯再度觀察的細胞結構部位,然後在樣本傳送過程中保持最佳的低溫冷凍條件下,安全將樣本無縫的傳送到電子顯微鏡.作該區域的電險成像.
THUNDER Imager EM Cryo CLEM 是新世代的低溫光學顯微鏡結合數位光電澄清影像與提升解析的解決方案, 利用 THUNDER, 可以把受測樣本區通過高解析度的影像澄清處理、即時去除焦平面外的模糊信號,從而對樣本細胞結構的特定選擇區域, 作更精確的確認須受低溫電顯再度觀察的細胞結構部位,然後在樣本傳送過程中保持最佳的低溫冷凍條件下,安全將樣本無縫的傳送到電子顯微鏡.作該區域的電險成像.
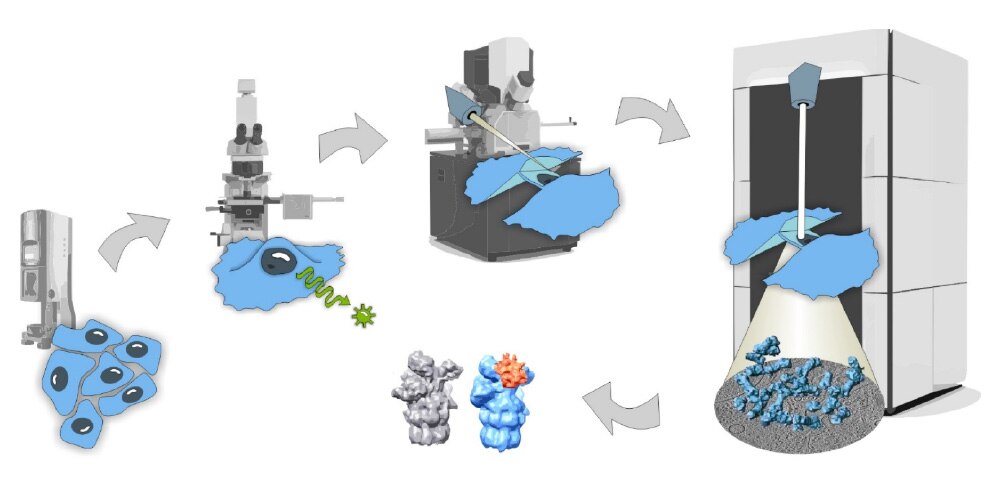
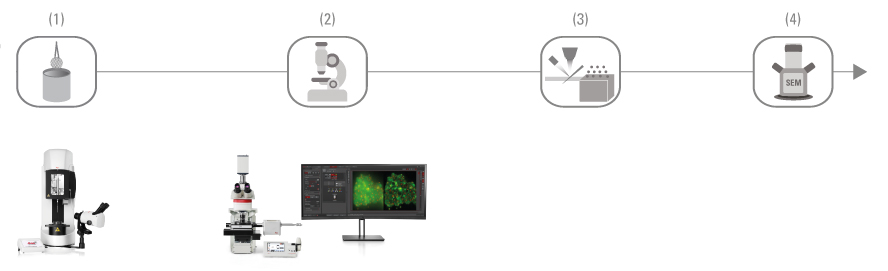
Cryo CLEM 工作流程
通過該工作流程,您可以分析冷凍切片樣本的高解析度超微結構。 對樣本進行高壓冷凍和冷凍切片。 之後,THUNDER EM Cryo CLEM 成像系統可説明您識別感興趣的區域,然後進行低溫透射電顯成像。 該工作流程適用於組織樣本。
(1) 高壓冷凍 (EM ICE)
(2) 冷凍切片 (EM UC7 / EM FC7)
(3) 識別感興趣的區域(THUNDER EM Cryo CLEM 成像系統)
(4) 低溫穿透電顯成像

保持冷凍條件
為了盡可能獲得成功的實驗結果,我們採用獨特的套筒系統和封閉式冷凍台, 確保樣本保持玻璃狀。 在裝載和傳輸乃至長時間圖像拍攝過程中,該系統可最大程度降低污染的可能性。
工作原理
使用套筒系統 (1) 可以安全快速地處理樣本。 壓夾機構可加快樣本通量以及將載網裝入冷凍台的時間。
傳送對介面 (2) 通過保持最佳溫度來保護冷凍樣本。
帶有同步物鏡蓋的冷凍台 (3) 將樣本保持在穩定的超壓下,從而防止樣本被空氣污染
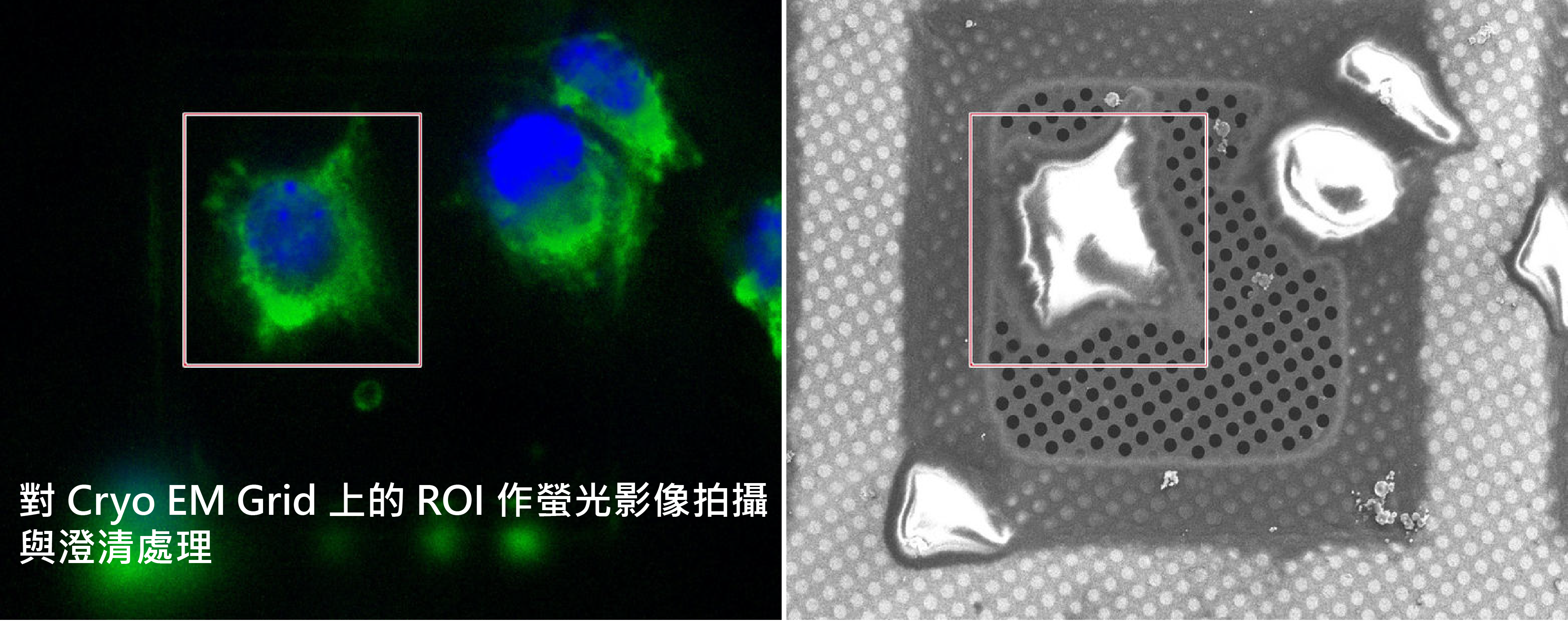
通過低溫光學顯微鏡的座標變換輕鬆檢索
THUNDER EM Cryo CLEM 成像系統集成的軟體不僅能夠引導您完成整個成像工作流程,而且您只需點擊一下即可匯出原始圖像資料和相關座標。 您可以立即在您指定的電子顯微鏡中重新定位細胞靶區,並開始研究樣本的超微結構。
上圖 : 選擇和檢索座標。 在電子顯微鏡載網上呈現並被選擇性標記的細胞螢光圖像。 利用座標標記,在 Thermo Scientific Aquilos 上呈現並精准再定位了同一個細胞。
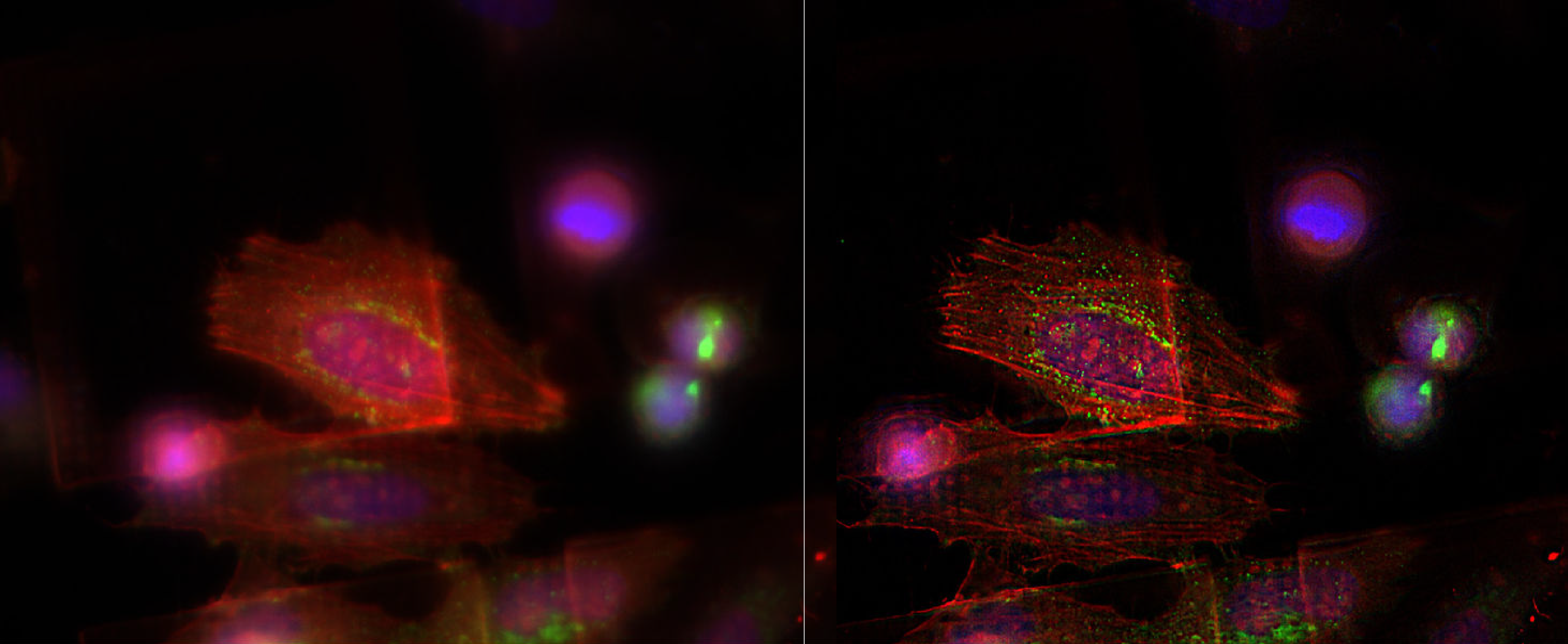
HeLa 細胞放在 Quantifoil R2 / 2 金膜的 G200F1 Grid上。
細胞使用 GFP-TGN46(標記反面高爾基網狀結構TGN)和 mCherry-Lifeact(標記纖維狀肌動蛋白)轉染。 細胞核使用 Hoechst 33342 染色。
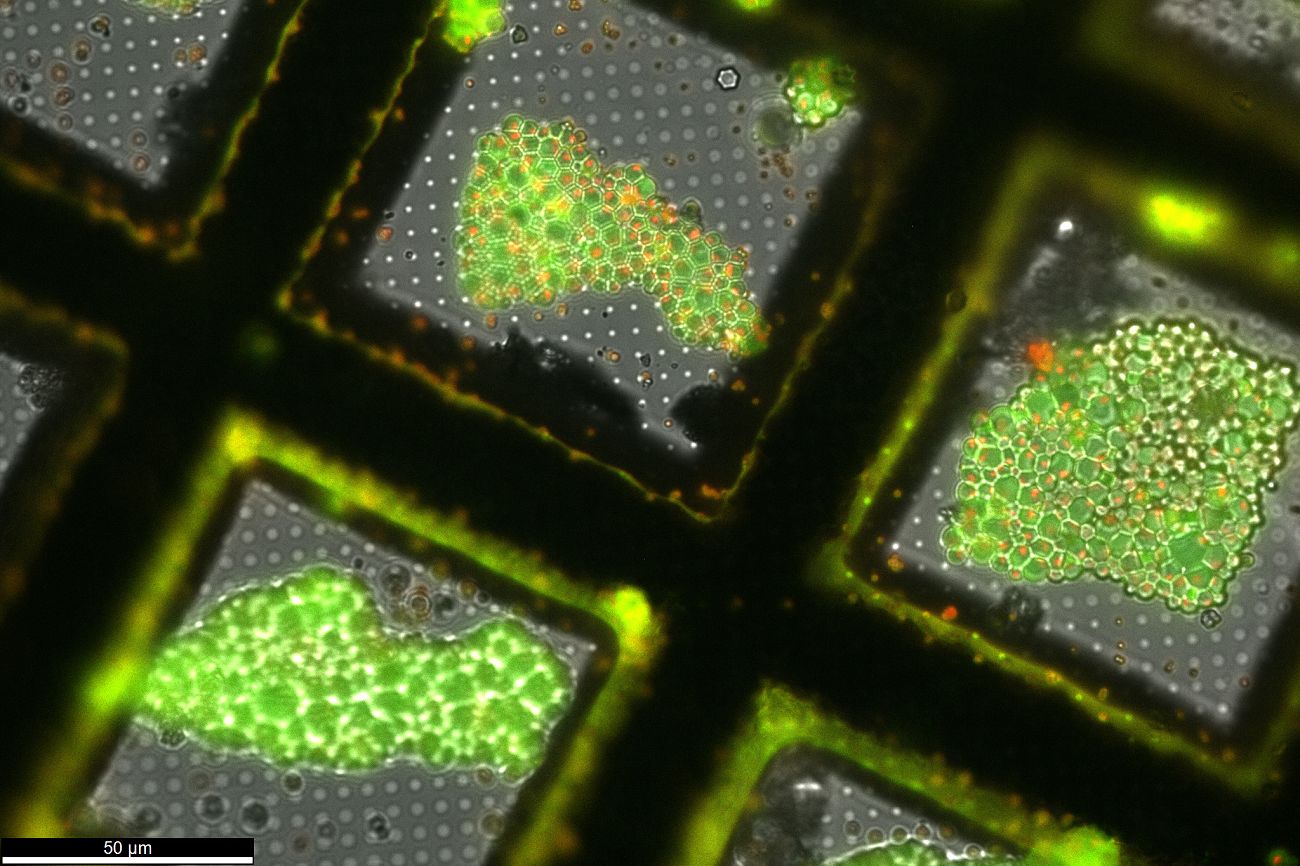
- Advanced cryo‐tomography workflow developments – correlative microscopy, milling automation and cryo‐lift‐out
J. KUBA, J. MITCHELS, M. HOVORKA, P. ERDMANN, L. BERKA, R. KIRMSE, J. KÖNIG, J. DE BOCK, B. GOETZE, A. RIGORT
https://doi.org/10.1111/jmi.12939 - Front Microbiol. 2017 Apr 11;8:596; Pleomorphism and Viability of the Lyme Disease Pathogen Borrelia burgdorferi Exposed to Physiological Stress Conditions: A Correlative Cryo-Fluorescence and Cryo-Scanning Electron Microscopy Study; Vancová M, Rudenko N, Vaněček J, Golovchenko M, Strnad M, Rego ROM, Tichá L, Grubhoffer L, Nebesářová J.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5387694/ - Nat Protoc. 2017 Jan;12(1):150-167; Correlated fluorescence microscopy and cryo-electron tomography of virus-infected or transfected mammalian cells; Hampton CM, Strauss JD, Ke Z, Dillard RS, Hammonds JE, Alonas E, Desai TM, Marin M, Storms RE, Leon F, Melikyan GB, Santangelo PJ, Spearman PW, Wright ER.
https://www.nature.com/articles/nprot.2016.168 - Methods Cell Biol. 2017;140:321-333; Matrix MAPS-an intuitive software to acquire, analyze, and annotate light microscopy data for CLEM; Schorb M, Sieckmann F.
http://www.sciencedirect.com/science/article/pii/S0091679X17300560 - Methods Cell Biol. 2017;140:303-320; triCLEM: Combining high-precision, room temperature CLEM with cryo-fluorescence microscopy to identify very rare events; Ader NR, Kukulski W.
http://www.sciencedirect.com/science/article/pii/S0091679X17300535 - Methods Cell Biol. 2017;140:85-103; A new method for cryo-sectioning cell monolayers using a correlative workflow; Kolovou A, Schorb M, Tarafder A, Sachse C, Schwab Y, Santarella-Mellwig R.
http://www.sciencedirect.com/science/article/pii/S0091679X17300559 - New hardware and workflows for semi-automated correlative cryo-fluorescence and cryo-electron microscopy tomography, Martin Schorba, b, Leander Gaechterc, Ori Avinoama, Frank Sieckmannd, Mairi Clarkea, Cecilia Bebeacuaa, e, Yury S. Bykova, Andreas F.-P. Sonnena, f, Reinhard Lihlg, John A.G. Briggsa, e, f,, a Structural and Computational Biology Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany, b Electron Microscopy Core Facility, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany, c Leica Microsystems (Schweiz) AG, Max Schmidheiny-Strasse 201, 9435 Heerbrugg, Switzerland, d Leica Microsystems GmbH, Am Friedensplatz 3, 68165 Mannheim, Germany, e Cell Biology and Biophysics Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany, f Molecular Medicine Partnership Unit, EMBL/Universitätsklinikum Heidelberg, Heidelberg, Germany, g Leica Mikrosysteme GmbH, Hernalser Hauptstraße 219, 1170 Vienna, Austria,http://www.sciencedirect.com/science/article/pii/S1047847716301356
- Correlative cryo-fluorescence and cryo-scanning electron microscopy as a straightforward tool to study host-pathogen interactions, Martin Strnad1,2, Jana Elsterová1,2,3, Jana Schrenková1,2, Marie Vancová1,2, Ryan O. M. Rego1,Libor Grubhoffer1,2 & Jana Nebesářová1,2,4, 1Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branišovská 31, České Budějovice CZ- 37005, Czech Republic. 2Faculty of Science, University of South Bohemia in České Budějovice, Branišovská 1760, České Budějovice CZ-37005, Czech Republic. 3Department of Virology, Veterinary Research Institute, Brno CZ-62100, Czech Republic. 4Faculty of Science, Charles University in Prague, Viničná 1594/7, Praha CZ-12800, Czech Republic. Correspondence and requests for materials should be addressed to M.S. (email: martin.strnad.cze(at)gmail.com),
https://www.nature.com/articles/srep18029 - Metskas LA, Briggs JAG
Fluorescence-Based Detection of Membrane Fusion State on a Cryo-EM Grid using Correlated Cryo-Fluorescence and Cryo-Electron Microscopy
Microscopy and Microanalysis. 2019 August; 25(4):942-949.
https://www.cambridge.org/core/journals/microscopy-and-microanalysis/article/fluorescencebased-detection-of-membrane-fusion-state-on-a-cryoem-grid-using-correlated-cryofluorescence-and-cryoelectron-microscopy/4933BFD9777A1E735075BFE5A60DFC90#fndtn-information





